Thermochemistry
Thermochemistry Summary
Thermochemistry is a mobile iOS app in Education by Roman Volinsky. Released in Mar 2016 (9 years ago). Store metadata: updated Oct 11, 2024.
Store info: Last updated on App Store on Oct 11, 2024 .
0★
Ratings:
Screenshots





App Description
Thermochemistry helps in evaluation of enthalpy or heat release/absorption of a system undergoing numerous temperature changes and phase transitions. The calculations take advantage of known values of heat capacity at constant pressure (Cp) and molar or per gram enthalpy of phase transition. Amount of compound can be defined in grams or moles, in a way that Cp and enthalpy units would match.
App provides enthalpy values for each step. Negative enthalpy points to exothermic process – heat release, while positive one to endothermic - heat absorption. °C and K are interchangeable.
Calorimetry section provides means for evaluation of the heat capacity of calorimeter and for finding equilibrium temperature of mixed system. Forward arrow button sets the final temperature of the mixture. Backward arrow button sets missing temperature or heat capacity of one of the components. Enthalpy values show heat flow for each component.
Example of problems solved by application (screenshots):
Problem 1: Calculate the amount of energy required to change 100.0 g of ice at -15.0 °C to steam at 125.0 °C. Known values:
Heat of melting = 334.16 J g¯1
Heat of vaporization = 2259 J g¯1
specific heat capacity for solid water (ice) = 2.06 J g¯1 K¯1
specific heat capacity for liquid water = 4.184 J g¯1 K¯1
specific heat capacity for gaseous water (steam) = 2.02 J g¯1K¯1
Solution:
1) Heating of 100.0 g of ice from -15.0°C to 0.0°C:
(100.0 g) (15.0 K) (2.06 J g¯1 K¯1) = 3090 J
2) Melting of 100.0 g of ice:
(100.0 g) (334.16 J g¯1) = 33416 J
3) Heating of 100.0 g of liquid water from zero to 100.0 Celsius:
(100.0 g) (100.0 K) (4.184 J g¯1 K¯1) = 41840 J
4) Evaporations of 100.0 g of liquid:
(100.0 g) (2259 J g¯1) = 225900 J
5) Heating of 100.0 g of steam from 100.0 to 125.0 Celsius:
(100.0 g) (25.0 K) (2.02 J g¯1 K¯1) = 5050 J
6) Summation of the results:
3090 + 33416 + 41840 + 225900 + 5050 = 309.3 kJ
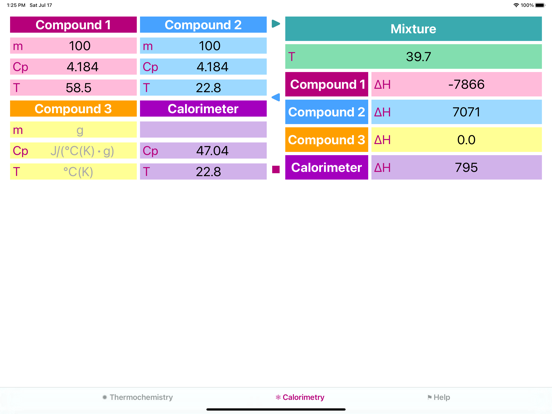
Problem 2: Determine the heat capacity of a coffee-cup calorimeter. During calibration 100.0 g of water at 58.5 °C has been added to 100.0 g of water, already in the calorimeter, at 22.8 °C. Calculate the heat capacity of the calorimeter in J/°C, if final temperature of the water is 39.7 °C. (Specific heat of water is 4.184 J/g °C.)
Solution:
1) Heat given up by warm water:
q = (100.0 g) (18.8 °C) (4.184 J/g °C) = 7865.92 J
2) Heat absorbed by water in the calorimeter:
q = (100.0 g) (16.9 °C) (4.184 J/g °C) = 7070.96 J
3) The difference was absorbed by the calorimeter:
7865.92 - 7070.96 = 794.96 J
4) Calorimeter constant:
794.96 J / 1